- HOME
- Notification: AMDD Recommends GS1 Standards for RFID Tags
Notification: AMDD Recommends GS1 Standards for RFID Tags
AMDD recommends its members to adopt GS1 standards for “encoding of information” of radio frequency identification (RFID) tags onto medical device and to use the UHF spectrum for “radio frequency for transmission.”
RFID, non-contact information recognition and matching system, is gaining momentum across various industries, including the medical device industry. It is expected that utilization of RFID will streamline processes across medical devices manufacturers, marketers, agents, dealers, as well as healthcare institutions. Also, use of RFID will secure patients access and higher safety.
Healthcare institutions, which use medical devices, and distributors/agents, who need to distinguish among various products from multiple producers, will benefit from an aligned “encoding of information” and “radio frequency for transmission.” Therefore, AMDD set up a RFID Working Group in October 2018 to discuss and decide on the aligned “encoding of information” and “radio frequency for transmission” regarding RFID tags, incorporating 23 AMDD member companies (see attachment 1). In March 2019, the Working Group concluded that “AMDD is to recommend GS1 standards and UHF radio waves.” The conclusion was submitted to and approved by the Executive Committee during the same month.
RFID tag format
| Encoding information | Merchandise information – SGTIN96 or SGTIN198 Lot #, expiry date |
|---|---|
| Radio frequency for transmission | UHF |
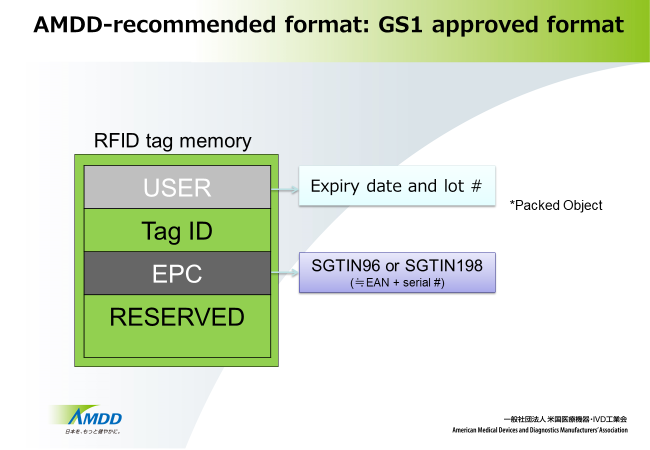
Henceforth, medical device manufacturers, marketers, agents, dealers, and healthcare institutions are recommended to adopt a unified approach by implementing the above format when introducing RFID tags.
If there are any questions regarding RFID tags on individual products, please direct such questions to the company that installed the tags (medical device manufacturers, marketers, agents, dealers, or healthcare institutions).
AMDD RFID Working Group Leader
Shigeyuki Masukawa
AMDD RFID Working Group Member companies (listed in random order)
- Arthrex Japan G.K.
- Abbott Japan Co., Ltd.
- Johnson & Johnson K.K.
- Cardinal Health Japan G.K.
- Edwards Lifesciences Ltd.
- Zimmer Biomet G.K.
- Cook Japan, Inc.
- Medicon Inc.
- STERIS Japan Inc.
- Smith & Nephew KK
- Medtronic Japan Co., Ltd.
- MicroPort Orthopedics Japan K.K.
- Century Medical, Inc.
- Baxter Limited
- Masimo Japan Corporation
- Nippon Covidien Ltd.
- Boston Scientific Japan K.K.
- Stryker Japan K.K.
- Alcon Japan Ltd.
- SEAOS, Inc.
- SATO HEALTHCARE CO., LTD.
- NTTDATA Corporation
- NTT Logisco Inc
*AMDD Associate Members

